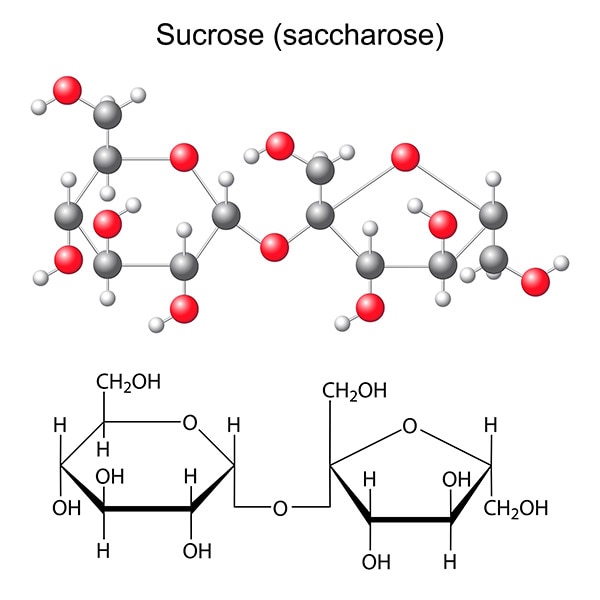
Sucrose
Table sugar is sucrose, synthesised by plants such as sugar cane and sugar beet. In chemical terms, sucrose is a disaccharide (‘double’ sugar) formed from a glucose molecule and a fructose molecule. Consumption of sucrose increased from the 19th century onwards, partly due to its widespread use in the agri-food industry.
A disaccharide on the dining table
Sucrose is a disaccharide commonly known as ‘table sugar’ or quite simply as ‘sugar’, comprising two monosaccharides, fructose and glucose. The carbohydrates synthesised in leaves travel as sucrose to the plant’s roots and other organs that are incapable of photosynthesis.
The date palm, sweet sorghum and Canadian maple are all sources of sucrose, however most of the sugar produced today comes from two plants: sugar cane, whose stem is 15 to 20% sugar, and sugar beet, whose root is between 14% and 19% sugar.
In 2015, around 166 million tonnes of sugar were produced worldwide, equivalent to 33 times the weight of the Great Pyramid of Giza in Egypt. Average consumption per capita varies greatly around the world, ranging from 15 kg in Africa to 53 kg in South America, according to the statistics for 2012. In Switzerland and in Europe in general, annual sucrose consumption went up from 3 kg per capita in 1850 to 40 kg in 2009.
Hydrolysed sucrose
During the first half of the 20th century, around 75% of all sugar was directly used in households, versus 25% used in industry. Today, the ratio is the other way round, as 85% of sugar is used in industrially processed food products versus just 15% used directly by households. The agri-food industry has thus become the biggest consumer of sucrose and derivatives such as inverted sugar, obtained by hydrolysis using an enzyme called invertase, produced by yeasts. In terms of chemistry, this runny syrup is an equimolecular mixture of fructose and glucose, meaning it contains equal amounts of both molecules. From a sensory point of view, it has great sweetening power and does not crystallise as easily as sucrose. It remains soft and smooth as it absorbs moisture and does not dry out. Manufacturers make use of these properties to produce softer products, reduce cooking time and stabilise ice creams and sorbets. Inverted sugar is mainly used in the pastry and confectionery industry and by professional chefs. It is only rarely available in retail outlets.
Nutrition
Sucrose, glucose and fructose all have the same calorific value of 4 kcal per gram.
Humans and bees produce inverted sugar naturally
The body cannot assimilate sucrose as it is. During digestion, an intestinal enzyme (invertase) hydrolyses sucrose, breaking down this disaccharide into two monosaccharides, fructose and glucose. Thus, our digestive system turns sucrose into inverted sugar in order to be able to absorb it. Fructose and glucose pass through the intestinal wall and enter the bloodstream. They then travel to the liver to be metabolised. In other words, they are released into the blood for immediate energy, or stored as glycogen or fatty acids for later use. Bees produce honey, which is also a natural form of inverted sugar. They produce invertase and regurgitate it to add it to the nectar. This enzyme transforms the nectar, essentially made up of water and sucrose, into glucose and fructose.
GARRET, Reginald H. GRISHAM, Charles M., 2000. Biochimie. Torino: De Boeck Université.
REECE, URRY, CAIN, WASSERMAN, MINORSKY, JACKSON, 2012. Biologie. Canada. Pearson
MARIA MERKI, Christophe, 2014. Sucre. Dictionnaire historique de la Suisse. Disponible à l’adresse :
http://www.hls-dhs-dss.ch/textes/f/F26241.php
SCHLIENGER, J.-L. et MONNIER, L. Du «sel indien» au High Fructose Corn Syrup Histoire du sucre des origines à nos jours. Médecine des maladies Métaboliques, 2012, vol. 6, no 5, p. 451-455.
BLAUER, R. Sugar World markets and trade. United States Department of Agriculture Foreign Agricultural Service, GAIN, report November, 2016.
https://apps.fas.usda.gov/psdonline/circulars/sugar.pdf (accédé le 20.02.2016)
OECD/FAO, “Sugar”, in OECD-FAO Agricultural Outlook 2016-2025, OECD Publishing, Paris. 2016 http://dx.doi.org/10.1787/agr_outlook-2016-9-en